A 1,2-Addition Pathway for C(sp2)—H Activation at a Dinickel Imide
Published in Chemistry – A European Journal, 2017
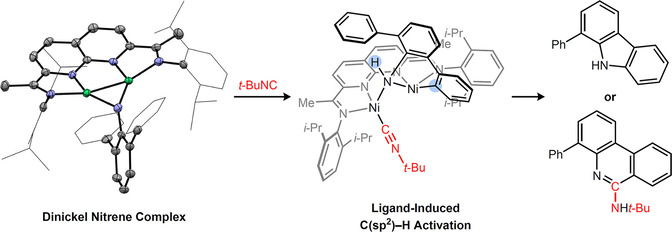
Abstract
A dinickel imido complex was synthesized using a redox-active naphthyridine-diimine supporting ligand. Upon coordination of an external ligand, the Ni2 core was disrupted, triggering an aromatic C-H activation reaction to generate a Ni2 (μ-NHAr)(Ar) species. This intermediate is capable of liberating free carbazole and phenanthridine products upon heating or treatment with excess tBuNC. Collectively, these studies establish a kinetically facile 1,2-addition mechanism for C(sp2 )-H activation, taking advantage of cooperative reactivity between two Ni centers.
Recommended citation: Powers I.G., Kiattisewee C., Mullane K.C., Schelter E.J., Uyeda C.† (2017). "A 1,2-Addition Pathway for C(sp2)—H Activation at a Dinickel Imide." Chemistry – A European Journal. 23(32):7694-7697. PMID: 28453895
